Two Elements That Have Similar Properties to Calcium
Both are alkaline earth metal. Both have same oxidation states 2 both are strong basic oxide.

Combination Reaction Reactions Combination Chemistry
Their oxides are alkaline 3.

. The columns are called groups. Two things that governments can do to create greater equality are. As you descend the Group the metal the element becomes more reactive.
The elements calcium strontium and barium were put in one group and family on the basis of their similar properties. As both element are placed in the same group they have same physical and chemical properties. Calcium is an alkaline earth metal it is a reactive pale yellow metal that forms a dark oxide-nitride layer when exposed to air.
Calcium shares properties with Magnesium and Strontium. Select two elements that have chemical properties similar to lithium. The nurse is teaching a patient about the anticholinergic agent prescribed for urinary retentionWhich statement by the patient indicates a need for further teaching.
Predict the group of elements and their number of valence. Calcium is a chemical element with the symbol Ca and atomic number 20. Lithium and Potassium 61 8.
Chemistry questions and answers. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. Lithium sodium and potassium elements were put in one group on the basis of their similar properties.
Select two elements that have chemical properties similar to lithium. All are metals 2. Its physical and chemical properties are most similar to its heavier homologues strontium and barium.
Initially the communist forces looked to be the weaker of the two and the Whites made good progress pushing inwards from the north south and east. Elements that appear close to each other on the table are related and tend to share many common properties. Strontium magnesium calcium beryllium.
What are two elements that have similar properties to the element calcium. The elements calcium strontium and barium were put in one group and family on the basis of their similar properties. What were those similar properties.
Why is the atomic number better than the atomic mass for organizing the elements in the periodic table. 5 Which of these sets of elements have similar physical and chemical properties. Mathematics 13022021 0950.
Oxygen nitrogen carbon boron B. Electro-negativity of Calcium and barium are approximately same. As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air.
As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Two factors that improve unemployment inflation and economic growth simultaneously are. Its physical and chemical properties are most similar to its.
Its physical and chemical properties are most similar to its heavier homologues strontium and barium. Which element listed should have chemical properties similar to fluorine F ALi BSi CBr DNe Br along with F a halogen column 7 of the periodic table Look at the periodic table. Magnesium calcium potassium and sodium appear tightly clustered in the periodic table.
Strontium Magnesium Calcium Beryllium 61 7. Medium Solution Verified by Toppr Those similar properties are- 1. As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air.
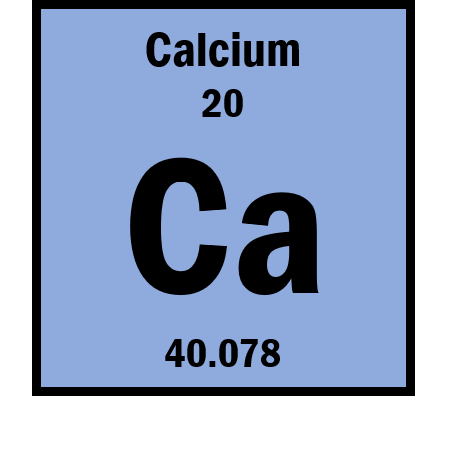
Magnesium Helium Strontium Calcium Potassium Sodium Boron Beryllium Carbon. Which elements have properties similar to calcium. Calcium is a chemical element with the symbol Ca and atomic number 20.
Its physical and chemical properties are most similar to its. The other metals in Group I are lithium potassium rubidium and francium. Calcium and Barium are placed in the same group 2 in the periodic table.
As an alkaline earth metal calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Calcium is a chemical element with the symbol Ca and atomic number 20. Name two elements that have properties similar to those of the element sodium.
Given that these four elements are so chemically similar it is not surprising that they function in your body together as electrolytes. Calcium is a chemical element with the symbol Ca and atomic number 20. What were those similar properties.
Each has valency 2.

Metals And Their Properties Metals Have Distinctive Properties Such As 1 Electrical Conductivity 2 Good Therm Gcse Science Teacher Notes Class Participation

Periodic Table Worksheet Answer Key Chemistry Worksheets Periodic Table Atomic Structure

Chemical Properties Of Calcium

The Elements Calcium Strontium And Barium Were Put In One Group Or Family On The Basis Of Their Similar Properties Which Were Those Similar Properties

Learn Chemistry With This Periodic Table Study Guide Study Guide Chemistry Chemistry Lessons

Calcium Definition Properties Compounds Britannica
Calcium Chemical Element Structure Reaction Water Uses Elements Examples Metal Gas

Helium Chemical Properties Uses Atomic Number Periodic Table Physical And Chemical Properties Helium Chemical

What Are The First 20 Elements Alkaline Earth Metals Rocks And Minerals Calcium
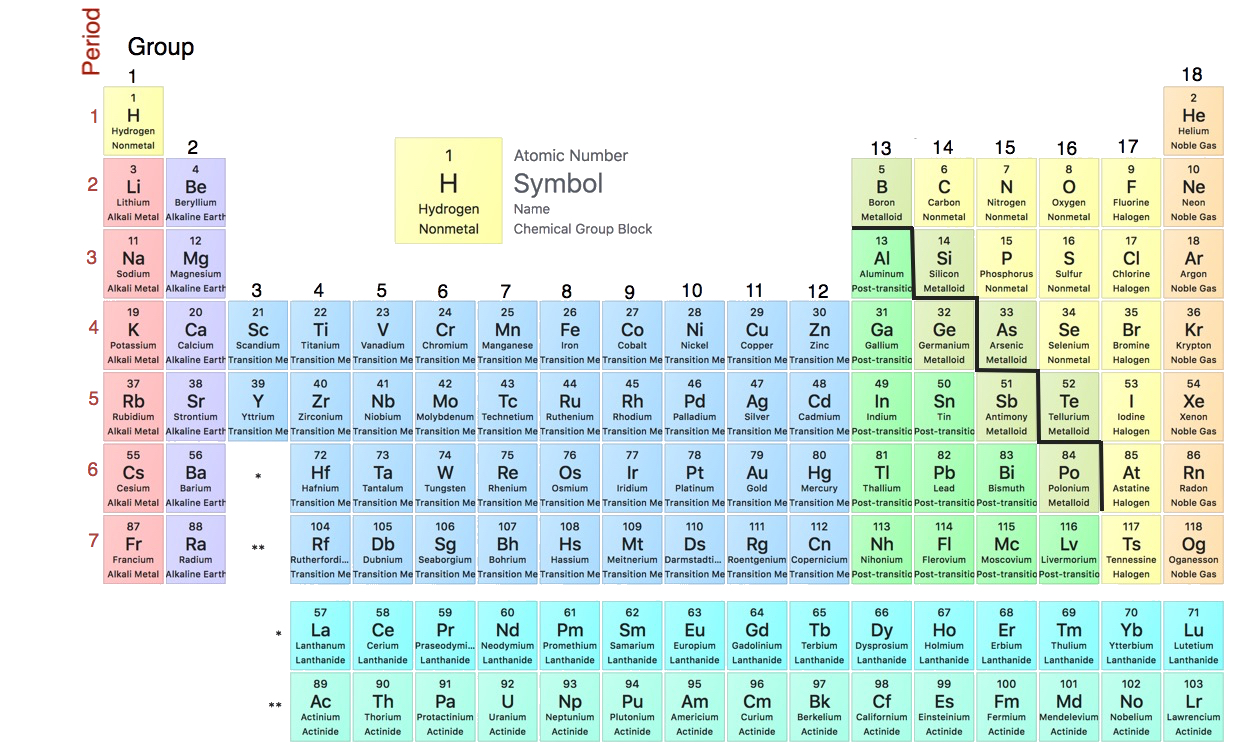
2 7 The Periodic Table Chemistry Libretexts

Chapter 7 Periodic Properties Of The Elements Electron Configuration Ionization Energy Electron Affinity
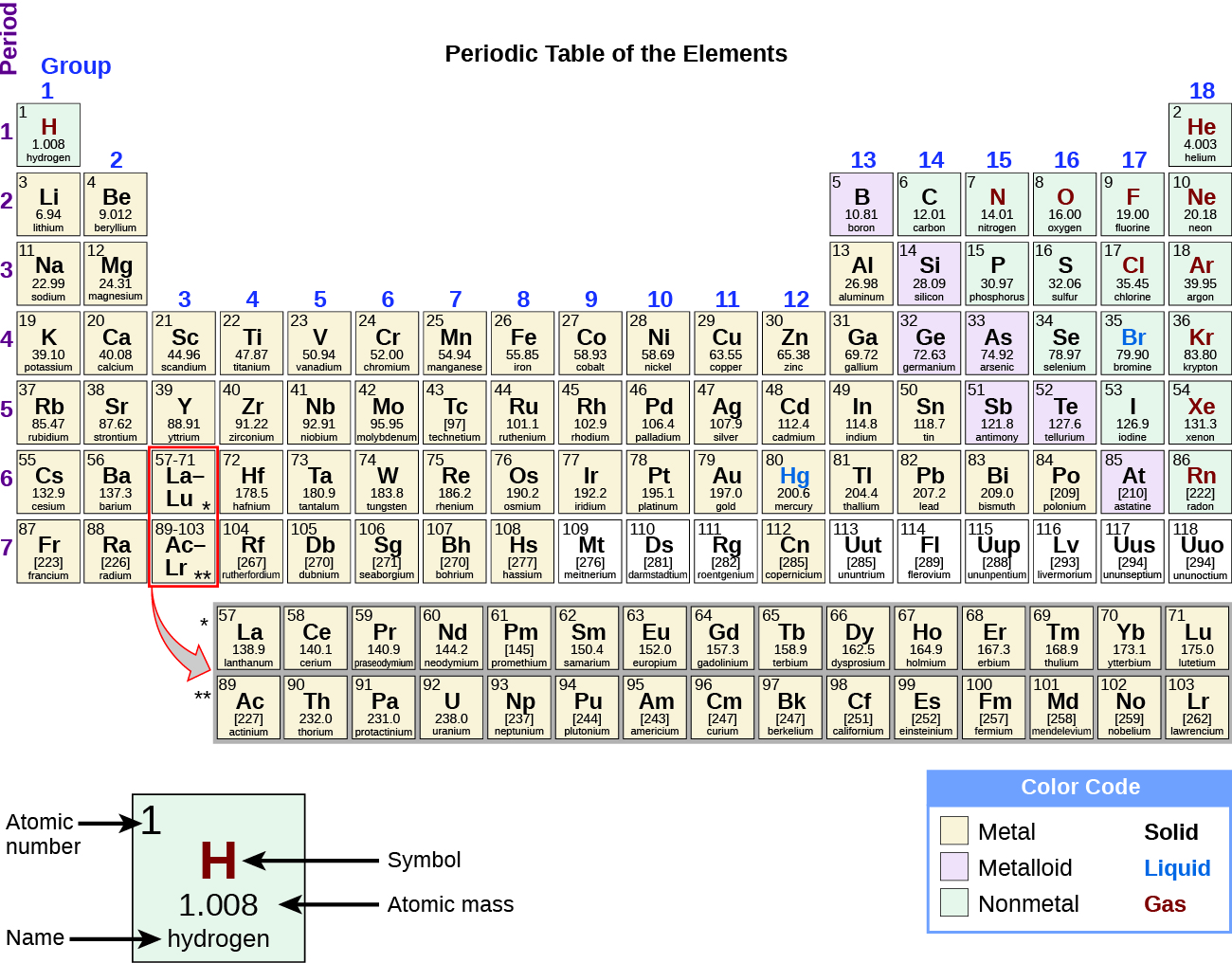
2 5 The Periodic Table Chemistry

Wonderful Life With The Elements Its A Wonderful Life Wonder High School Chemistry

What Is A Binary Compound Definition And Examples Covalent Bonding Compounds Binary

Alkaline Earth Metal Properties List Reactivity Britannica

Group 2 Elements On The Periodic Table Names Properties Activity Video Lesson Transcript Study Com

Here S What The Chemical Elements Look Like In Pure Form Periodic Table Of The Elements Cool Things To Buy Element

Comments
Post a Comment